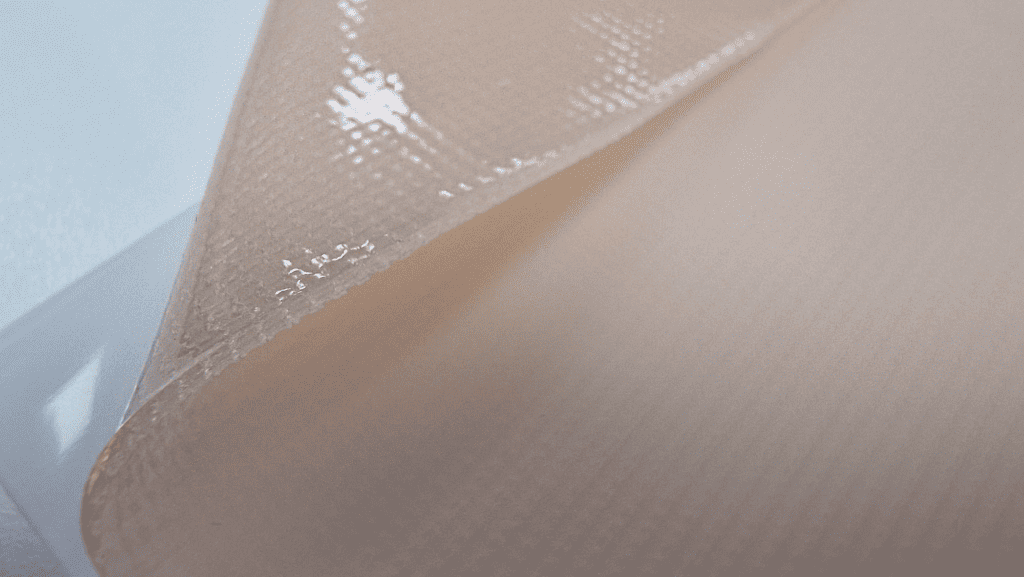
Key considerations
When manufacturing a hydrogel dressing, it is essential to consider various factors to ensure the production of a high-quality product that meets safety and efficacy standards. Here are some key considerations to keep in mind:
- Material Selection: Choose biocompatible and non-toxic materials that are suitable for the intended application. Ensure that the selected materials possess the necessary properties for maintaining moisture, promoting healing, and adhering to the wound bed appropriately.
- Formulation Optimization: Develop an optimized formulation that balances the desired properties of the hydrogel, such as water content, mechanical strength, biodegradability, and biocompatibility. Conduct thorough research and development to achieve the optimal balance between these properties.
- Manufacturing Process Control: Implement robust manufacturing processes with strict control over parameters such as temperature, pH, mixing time, and curing conditions. Ensure consistent quality through rigorous process validation and control measures.
- Sterility Assurance: Establish a sterile manufacturing environment and adopt aseptic techniques to prevent contamination during the production process. Implement stringent quality control measures to ensure that the final product is free from harmful microorganisms.
- Regulatory Compliance: Adhere to regulatory guidelines and standards specific to the production of medical devices and wound care products. Ensure that the manufacturing process complies with Good Manufacturing Practices (GMP) and other relevant industry regulations.
- Product Stability and Shelf Life: Conduct comprehensive stability studies to assess the product’s shelf life and performance over time. Ensure that the hydrogel dressing remains stable under various storage conditions to maintain its efficacy and integrity throughout its shelf life.
- Quality Control and Assurance: Implement a comprehensive quality control system to monitor every stage of the manufacturing process, from raw material procurement to the final product packaging. Conduct rigorous quality testing, including mechanical testing, biocompatibility assessments, and sterility testing, to guarantee product safety and efficacy.
- Scalability and Cost-Effectiveness: Develop a manufacturing process that is scalable to meet market demand while maintaining product quality and consistency. Balance the need for scalability with cost-effectiveness to ensure the production of a competitive and commercially viable hydrogel dressing.

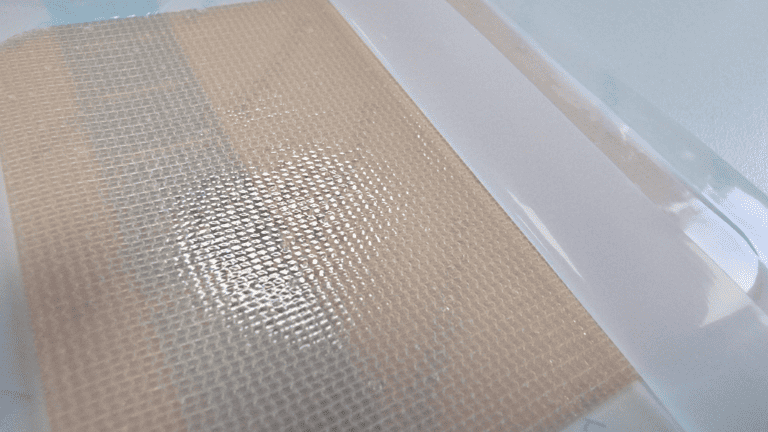
By carefully considering these factors during the manufacturing process, you can produce a high-quality hydrogel dressing that meets the necessary standards for safety, efficacy, and performance in clinical applications. Regular review and optimization of the manufacturing process based on new research and technological advancements will help maintain a competitive edge in the market.

Read More from PolarSeal
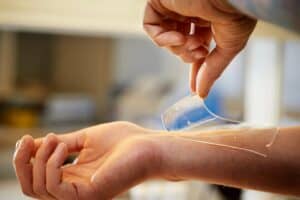
A closer look at hydrogels
What is a hydrogel? Hydrogel is a network of polymer chains that are hydrophilic, sometimes found as a colloidal gel in which water is the
The Importance of Collaboration in Medical Device Manufacturing.
In the dynamic world of healthcare, technological advancements are transforming patient care and treatment methods. One of the driving forces behind these transformations is the

A Bespoke Solution For A Global Medical Device Manufacturer For An Advanced Wound Care Dressing.
Introduction With the wound care market growing and predicted to continue to expand over the next few years, global medical device manufacturers contact PolarSeal to