Route to market for medical devices
Quality and Regulatory getting you market ready
PolarSeal® equips you with everything you need to take your medical device to market within all territories.

Tailored pathways
Every project and every medical device is different with unique requirements intended for particular markets. PolarSeal® understands these variations and regulatory requirements, as well as the barriers encountered to taking your product to market. We work with you no matter the stage of the process that you are in. Our experienced teams will collaborate with you to achieve the desired outcome, right from initial prototyping through to testing, packaging, and product certification.
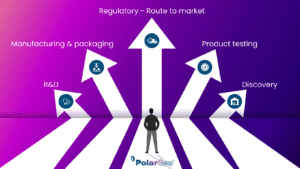
A typical full end-to-end process would consist of:
- Project discovery
- Research and development
- Manufacturing and packaging
- Product testing
- Regulatory - route to market



Project discovery
From the initial concept through to supply chain management, PolarSeal® provides a solution to fulfil your entire project expectations from beginning to end.
Customers can enter the cycle at any point and let PolarSeal® manage the project going forward with clear, concise, and continuous communication.
- Device classification (Class I, IIA, IIB)
- Intended use
- Materials selection
- Prototyping
- Packaging/Labelling requirements
- Testing requirements
- Sterile/Non-sterile
- Project planning
Research & development
PolarSeal® take the time to understand your device and its intended use within the market, that is why we guide you through the R&D process of material and adhesive selection through to device realisation through prototyping. We create a solution for you from start to finish and beyond.
- Materials selection
- Device prototyping
- Design control support/Design dossiers
- Design verification/validation
- Packaging solutions
- Labelling design


Manufacturing & packaging
PolarSeal® can perform contract manufacturing and converting services within our cleanroom facilities, taking your final product through packing and pouching as well as sterilization, providing a pre-market readiness solution.
- ISO 7 cleanrooms
- ISO 13485
- Process validation
- PFMEA
- Packaging validation
- Sterilization validation
Product testing
PolarSeal® performs in-house testing to demonstrate device reliability and safety, ensuring the selected materials and adhesives are also the right combination for your application and right for the intended end user.
- Performance testing device and packaging
- Transit testing
- Performance tests (base layer adhesion/peel testing)
- Stability testing
- Packaging integrity testing
- Biological Safety Evaluation of Materials


Regulatory - route to market
There are multiple routes to market for medical device companies, PolarSeal® assists in this process helping you with classification, document preparation and final submission.
- UK Responsible Person Services (UKRP)
- CE Technical Documentation & Design Dossiers
- MDR technical file compilation (EU 2017/745)
- UKCA Marking
- CE Marking
- Clinical Evaluation Report (CER) Writing
- Risk Management
- Post-Market Clinical Follow-up protocol (PMCF)
- Periodic Safety Updated Report (PSUR)
- US – FDA 510(k)s / De Novo Submissions
Get in touch
Click below to make an enquiry to discuss your project requirements in more detail.