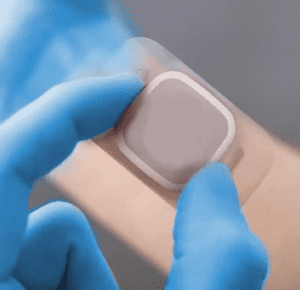
PolarSeal are proud to announce during our recent Audit this month undertaken by the LRQA that we have now been certified to the new standards; ISO13485:2016 and IS09001:2015. We have also been recertified as compliant with the MDD (medical device directive) 93/42/EEC and UK Medical Device Regulations.
(To view our updated certification, please click: ISO 134845:2016 and ISO9001:2015)
ISO13485:2016 Specifies requirements for a quality management system where an organisation needs to demonstrate its ability to provide medical devices to consistently meet customer and regulatory requirements.
For more info on ISO13485: https://www.iso.org/standard/59752.html
Organisations can be involved in multiple stages of the life-cycle from design and development, all the way through to distribution and installation of a medical device.
ISO9001:2015 is the international standard that specifies requirements for a quality management system (QMS). Organisations use the standard to demonstrate the ability to consistently provide products and services that meet customer and applicable statutory and regulatory requirements.
For more info on ISO9001: https://www.iso.org/standard/62085.html
CE Marking capabilities
This logo is placed on medical devices to show they conform to the requirements in the directives. It shows the device is fit for purpose stated and is that it meets the legislation relating to safety. The product can be freely marketed anywhere in the European Union.
See more: MDD directive – https://www.gov.uk/guidance/medical-devices-conformity-assessment-and-the-ce-mark
PolarSeal have top level experience in bringing products through the CE marking process. From the very beginning through to end-product.