The advent of Continuous Glucose Monitoring (CGM) patches has revolutionised diabetes management, offering patients a non-intrusive, real-time window into their glucose levels. This technology is a result of significant advancements in materials science, biomedical engineering, and patient-centric design.
In the rapidly evolving landscape of medical technology, PolarSeal® distinguishes itself through its expertise in developing and converting Continuous Glucose Monitoring (CGM) patch solutions.
Our focus lies in pioneering development and conversion methods for CGM patch solutions, contributing to significant advancements in the field. Our commitment to innovation and collaboration drives the evolution of CGM patch technology, facilitating breakthroughs that redefine diabetes management.
Key Takeaways:
- Innovative development process: Discover the step-by-step development and conversion methods of PolarSeal®’s CGM patch solutions, from the initial ideation phase to the final product.
- Material and design excellence: Understand the critical importance of selecting optimal materials and adhesives, ensuring that each CGM patch offers superior performance, durability, and user comfort.
- Strategic partnerships: Gain insights into PolarSeal®’s collaborations with leading raw material suppliers and service providers.
- Precision manufacturing: Explore advanced manufacturing techniques, including die cutting and assembly processes.
- Enhancing diabetes management: Recognise PolarSeal®’s commitment to improving the quality of life for individuals with diabetes through the development of wearable, user-centric CGM patches that stand out for their innovative features and design.
Continuous Glucose Monitoring (CGM) is an innovative tool that has revolutionised diabetes management. It provides real-time, dynamic blood sugar readings, offering individuals greater control over their condition. Accompanying these devices are CGM patches – unobtrusive adhesives that secure the CGM sensors to the skin.
A perfect blend of innovative materials, science, and user-centric design, the CGM patch is more than just a medical device; it represents a leap towards a future where managing diabetes is less intrusive and more integrated into the daily lives of those affected.
PolarSeal® holds extraordinary expertise in the development of these CGM patches, ensuring reliability and performance through features such as extended wear duration, unmatched comfort, superior moisture resistance, and optimal material conductivity.
This exploration delves into PolarSeal®’s CGM patch development and conversion methods, revealing the intricate processes that lead from conceptualisation to the final product. It showcases how the material selection, design, and manufacturing techniques coalesce to create a wearable reality for diabetes management, illustrating how PolarSeal® transformed a visionary idea into a wearable reality that enhances the quality of life for diabetes patients worldwide.
Ideation to Production: Understanding the Process of Developing CGM Patches
In recent years, there has been a surge in the development of wearable medical devices, and PolarSeal®’s CGM patch solution is at the forefront of this innovation. PolarSeal® uses cutting-edge technology to continuously monitor glucose levels in diabetes patients without the need for frequent finger pricks. This not only reduces discomfort but also provides more accurate and real-time data to patients and healthcare providers.
Through a series of strategic steps, including design brainstorming, material selection, validation and preparation of the production line for conversion, and assembly, PolarSeal® navigates the complex landscape of medical device manufacturing to ensure that the initial concept is both innovative and feasible for production.
The goal is to engineer a CGM patch that is not only robust and efficient in its technical aspects but also discreet and comfortable for long-term wear. With continuous feedback loops and iterative design thinking, PolarSeal®’s product development team works diligently to refine concepts before transitioning to the production stage.
By understanding the intricacies of this development and conversion process, we gain insight into how PolarSeal® navigates the challenges and opportunities inherent in creating wearable medical technology that is both effective and comfortable for everyday use. Let’s delve into the process.
The following sections will meticulously explore each of these critical stages, shedding light on the journey towards creating a device poised to revolutionise diabetes management.

Confirming Design and Bill of Materials (BOM) for CGM Patches
After finalising the initial CGM Patch concept, PolarSeal® confirms the design and Bill of Materials (BOM). This step is pivotal in understanding the specific components and materials required to produce the CGM patch.
The design phase involves creating detailed blueprints that outline the CGM patch’s architecture, from sensor integration to medical adhesive layers.
Selecting the BOM is a meticulous process. Each component, be it the breathable backing material or the skin-friendly adhesive, is chosen for its performance, durability, and compliance with stringent medical standards. PolarSeal® leverages cutting-edge technology and materials to ensure the CGM patch’s reliability and comfort.
The Bill of Materials (BOM) not only lists the materials used but also outlines their quantities, specifications, and suppliers, which the production phase can commence with all the necessary information.
PolarSeal® collaborates closely with clients and suppliers to finalise the specifications, ensuring that the end product meets both clinical and patient needs.

Selecting Optimal Materials and Adhesives for CGM Patches
Selecting the optimal materials and adhesives for CGM patches is a critical step in ensuring their effectiveness and user comfort. PolarSeal® employs a comprehensive evaluation process to identify materials that are not only durable and flexible but also hypoallergenic to minimise skin irritation.
The adhesives used must securely attach the CGM patch to the skin, allowing for continuous wear even during activities such as showering, swimming, and exercising. This selection process involves rigorous testing under various conditions to guarantee the CGM patches remain functional and comfortable over extended periods.
By prioritising these qualities, PolarSeal® aims to deliver a product that users can trust for accurate monitoring and minimal discomfort.
Sourcing Materials from Leading Raw Material Suppliers
PolarSeal® forges strategic alliances with premier raw material suppliers like 3M, FLEXcon, and Avery Dennison to procure top-tier components for its CGM patches.
Through these partnerships, PolarSeal® has access to cutting-edge materials and adhesives that comply with rigorous medical device standards, ensuring unwavering product quality and reliability. Furthermore, this collaboration fosters a culture of ongoing innovation, driving the evolution of future CGM patch developments.
By establishing dependable relationships with trusted suppliers, PolarSeal® secures a steady stream of medical materials, crucial for adhering to production schedules and meeting the demand in the medical market. This concerted effort guarantees that each material adheres to stringent standards for medical application, safeguarding the safety and efficacy of the final product for continuous glucose monitoring.
Confirm Tooling for Die Cut Parts in CGM Patch Manufacturing
Following the selection of ideal materials and adhesives, the next step is to confirm and procure precise tooling for die cut parts. Tooling for die cut parts is a critical manufacturing process for CGM patches, as it ensures the accuracy and uniformity of each component.
Leveraging advanced die cutting techniques, PolarSeal® meticulously crafts precise shapes and sizes for the CGM patches, accommodating the intricate designs necessary for optimal functionality. The selection of tooling for die cut parts entails meticulous analysis of the CGM patch design to determine the exact specifications required for the die cut machinery. This step is paramount not only for part accuracy but also for optimising production efficiency and minimising waste.
Once the die cut tooling specifications are confirmed, PolarSeal® orders custom tooling from specialised manufacturers, ensuring that every die cut part conforms to the precise specifications for the CGM patches. This meticulous attention to detail in the die cutting process is integral to PolarSeal®’s ability to deliver high-quality, reliable products to clients and end users.
Within its state-of-the-art ISO 7 class 10,000 cleanroom facilities, PolarSeal® ensures each patch is cut to exact specifications, guaranteeing the quality and performance of the final product.
After thorough validation and with full confidence in the quality and consistency of the output, the manufacturing teams proceed with the actual CGM patch conversion. Through this process, PolarSeal® demonstrates its unwavering commitment to delivering a reliable and high-quality product for continuous glucose monitoring.

The Conversion Process for CGM Patches
The conversion process transforms raw materials into finished CGM patches. This stage encompasses several steps, including die cutting, laminating, and assembling the various components.
PolarSeal®’s conversion process is carried out in state-of-the-art cleanroom environments to ensure the highest level of product purity and compliance with medical standards. The die cutting step is performed with precision, cutting the materials into specific shapes that form the foundation of the CGM patches.
Following die cutting, the materials undergo a lamination process, where different layers of the medical adhesive materials are fused to enhance the CGM patch’s durability and functionality. Lamination ensures that the CGM patches can withstand various environmental factors and remain adhered to the skin for the necessary duration.
The assembly of these components is the final step in the conversion process, where the CGM patches are put together in their final form. Each patch is inspected for quality assurance, guaranteeing that only products that meet PolarSeal®’s strict standards reach end users.
This meticulous conversion process is what allows PolarSeal® to provide reliable and comfortable CGM patches that positively impact diabetes management for users worldwide.
Explore our production process and quality control measures at PolarSeal® in greater depth.

Assembling Components for Complete CGM Patch Assembly
In the final assembly phase, all components necessary for the CGM patch’s functionality converge, forming a single unit. Utilising skilled technicians and cutting-edge assembly lines, PolarSeal® meticulously integrates the die cut parts, sensors, and electronics. Adhering to stringent quality control protocols, this process guarantees that each patch meets rigorous standards for functionality and reliability.
It’s important to highlight that our capabilities primarily revolve around converting and assembling stick-to-skin patches. We prioritise working with these materials and exclude the handling of hard plastics and similar materials.
Throughout this phase, every component, including sensors, adhesive substrates, and electrodes, undergoes meticulous integration to achieve the desired outcome. Following assembly, each patch undergoes a comprehensive series of final quality assessments to ensure it aligns with performance expectations and industry standards.
Our overarching objective is to produce a final product that not only functions optimally but also prioritises user comfort and discretion in the daily usage of CGM devices. Through this meticulous and iterative process, PolarSeal® strives to deliver CGM patches of the highest quality, ultimately aiming for user satisfaction and confidence in our products.
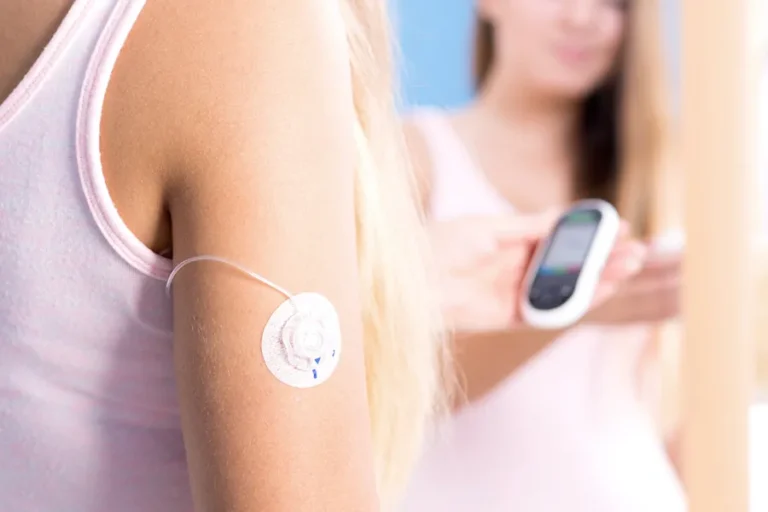
Key Features of PolarSeal®'s CGM Patches
Wear duration
Conformability
An essential aspect of any CGM patch is its ability to conform to the body’s contours. This ensures that it maintains contact with the sensor no matter how the skin moves. PolarSeal®’s CGM patches offer exceptional flexibility and adaptability, providing comfort without compromising the patch’s integrity or functionality.
Moisture resistance and MVTR
A key feature that makes CGM patches durable is their ability to resist moisture. PolarSeal®’s CGM patches boast high moisture vapour transmission rates (MVTR), meaning they allow the skin to breathe while protecting the CGM sensor from moisture-related damage. This attribute is particularly important for maintaining skin health and adhesive performance in humid environments or during physical activity.
Material conductivity
Connectivity between the sensor and the patch plays a vital role in the accuracy of glucose readings. PolarSeal® ensures that the materials used in their CGM patches have the necessary conductivity to transmit readings accurately, providing reliable results on which medical professionals and patients can depend.

CGM Patch Development with PolarSeal®
The development of CGM patches by PolarSeal® is a journey marked by innovation, precision, and a deep commitment to improving diabetes management. Each phase of the development process, from selecting materials to assembling the final product, is carried out with the utmost care, reflecting PolarSeal®’s dedication to quality and patient care.
PolarSeal®’s pioneering approach to CGM patch development is setting new standards in the medical device industry, offering patients a more comfortable and convenient way to monitor their glucose levels. As technology advances, PolarSeal® continues to explore new frontiers in medical device development, promising a future where managing chronic conditions like diabetes is more accessible and less burdensome.
Explore more about PolarSeal®’s solutions to wearable medical technology and CGM patches through their insightful case studies, detailed applications, and informative blog posts. Delve into the world of electro-medical converting and discover how PolarSeal® is leading the way in wearable medical device solutions.
Contact us today to learn more about our converting capabilities and services.
Read More from PolarSeal

How AI could advance the healthcare industry?
Artificial Intelligence, commonly referred to as ‘AI’ is a rapidly growing technology advancement whereby technology, i.e. computers, machines and devices are programmed with the ability

What to consider when classifying a medical device
Medical device classification is a regulatory process that categorizes medical devices into different classes based on their intended use, risks, and other relevant factors. The
7 Types of Diagnostic Products & Wearable Diagnostic Devices Important in the Personal Healthcare Industry
The medical industry is constantly evolving, and new diagnostic products and wearable diagnostic devices are key to keeping healthcare teams ahead of the curve and








